| Line 1: | Line 1: | ||
=Black Body= | =Black Body= | ||
| + | |||
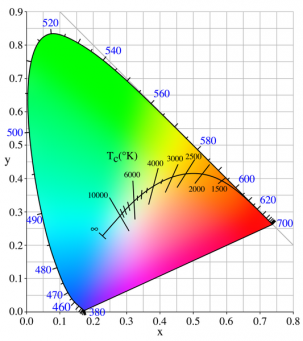
| + | [[File:PlanckianLocus.png|thumb|303px|The color (chromaticity) of blackbody radiation depends on the temperature of the black body; the locus of such colors, shown here in CIE 1931 x,y space, is known as the Planckian locus.]] | ||
| + | |||
| + | [[Image:Blackbody-colours-vertical.png|right|38px]] | ||
| + | |||
| + | |||
In terms of frequency (<math>\nu</math>) or wavelength (''λ''), Planck's law is written: | In terms of frequency (<math>\nu</math>) or wavelength (''λ''), Planck's law is written: | ||
:<math>B_\nu(T) = \frac{ 2 h \nu^{3}}{c^2} \frac{1}{e^\frac{h\nu}{k_\mathrm{B}T} - 1},</math> <math>\text{ or }\,</math> <math>B_\lambda(T) =\frac{2 hc^2}{\lambda^5}\frac{1}{ e^{\frac{hc}{\lambda k_\mathrm{B}T}} - 1}</math> | :<math>B_\nu(T) = \frac{ 2 h \nu^{3}}{c^2} \frac{1}{e^\frac{h\nu}{k_\mathrm{B}T} - 1},</math> <math>\text{ or }\,</math> <math>B_\lambda(T) =\frac{2 hc^2}{\lambda^5}\frac{1}{ e^{\frac{hc}{\lambda k_\mathrm{B}T}} - 1}</math> | ||
| Line 20: | Line 26: | ||
1931 Illuminants: | 1931 Illuminants: | ||
| − | * Illuminant A = Typical Incandescent Light | + | * Illuminant A = Typical Incandescent Light (2856 K) |
* Illuminant B = Direct Sunlight | * Illuminant B = Direct Sunlight | ||
* Illuminant C = Average daylight from total sky (ambient sky light) | * Illuminant C = Average daylight from total sky (ambient sky light) | ||
Revision as of 17:40, 29 December 2011
Black Body
In terms of frequency (<math>\nu</math>) or wavelength (λ), Planck's law is written:
- <math>B_\nu(T) = \frac{ 2 h \nu^{3}}{c^2} \frac{1}{e^\frac{h\nu}{k_\mathrm{B}T} - 1},</math> <math>\text{ or }\,</math> <math>B_\lambda(T) =\frac{2 hc^2}{\lambda^5}\frac{1}{ e^{\frac{hc}{\lambda k_\mathrm{B}T}} - 1}</math>
where B is the spectral radiance, T is the absolute temperature of the black body, kB is the Boltzmann constant, h is the Planck constant, and c is the speed of light. However these are not the only ways to express the law; expressing it in terms of wavenumber rather than frequency or wavelength is also common, as are expression in terms of the number of photons emitted at a certain wavelength, rather than energy emitted. In the limit of low frequencies (i.e. long wavelengths), Planck's law becomes the Rayleigh–Jeans law, while in the limit of high frequencies (i.e. small wavelengths) it tends to the Wien approximation.
(source http://en.wikipedia.org/wiki/Planck%27s_law)
RUFF STUFF
CCT = Correlated Color Temperature (6500K, etc.)
CIE Illuminants =>
- General lighting conditions (when taking a picture, or displaying one).
- Spectral characteristics similar to natural light sources
- Reproducible in the laboratory
1931 Illuminants:
- Illuminant A = Typical Incandescent Light (2856 K)
- Illuminant B = Direct Sunlight
- Illuminant C = Average daylight from total sky (ambient sky light)
1963 D Illuminants (more accurate daylight conditions):
- Illuminant D = Phases of daylight. Necessarily followed by the first 2 digits of the CCT (e.g. D65 = D 6504K)
- Represent daylight more completely and accurately than do Illuminants B and C because the spectral distributions for the D Illuminants have been defined across the ultraviolet (UV), visible, and near-infrared (IR) wavelengths (300–830 nm).
- Most industries use D65 when daylight viewing conditions are required
- D50 is used by graphic arts industry => more spectrally balanced across spectrum
Other illuminants:
- Illuminant E = Equal energy illuminant
- Illuminant F = Fluorescent lamps of different composition.
References
An Introduction to Appearance Analysis (2001) http://www.color.org/ss84.pdf